DTU Metabolomics Core
DTU Metabolomics Core provides analytical service to the researchers at DTU Bioengineering by grouping all major analytical instrumentation under a single management in dedicated laboratories. The main objective is to ensure an efficient use of all equipment, to maintain a high level of analytical skills and knowledge as well as secure cost-efficient operation of the very expensive equipment.
Our mission is to provide the researchers access to state-of-the-art analytical instrumentation and methodologies to detect all relevant secondary metabolites from biological system with an emphasis on microbial systems.
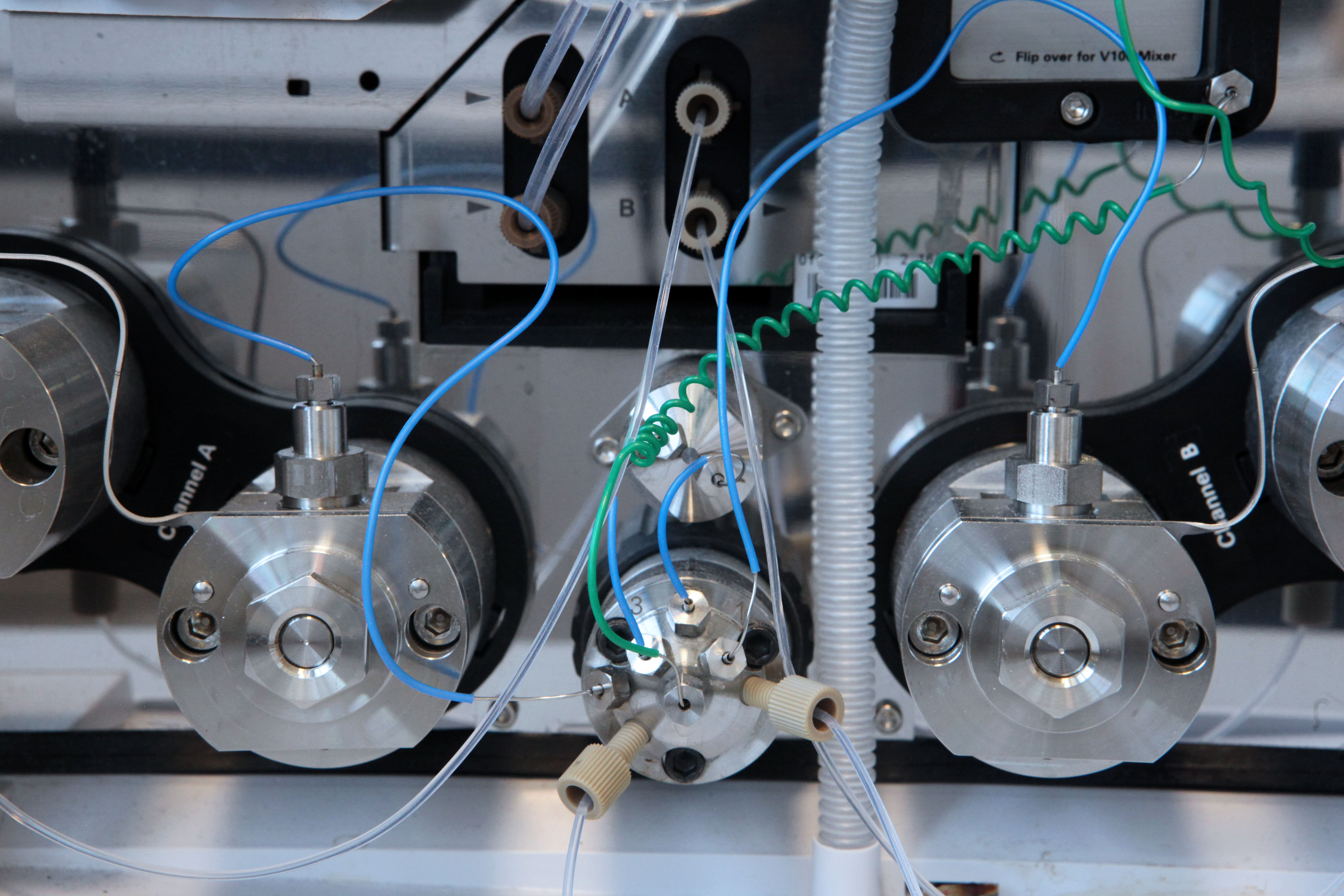
Advanced high resolution mass spectrometry combined with chromatography is a core part of all our work. We have a long history of work on secondary metabolite profiling from various microorganisms including both fungi and bacteria. We have a large collection of more than 1500 natural products and harbor an in-house mycotoxin MS/MS library with 500 reference standards. Here we provide cutting-edge dereplication (see 1, 2,3) for novelty evaluation of secondary metabolites in e.g. drug discovery applications, now also including coupling this with MS/HRMS libraries and stable isotope-labelling for precursor detection. This also involves many projects on characterization of biosynthetic pathways of polyketides (1) and non-ribosomal peptides (2).
The complex mixture of microbial metabolites, together with the more complex media components interfere with mass spectrometric detection. To reduce the matrix effects, we have a long tradition of working with sample preparation especially solid phase extraction (1,2,3,4).
Getting accessThe core is accessible to all researchers at DTU, after consultation with Metabolomics Core staff. Before being allowed to use instruments, please pay attention to the following points:
- How should the sample preparation be done? To ensure not contaminating the instruments and obtaining valid data, please ask before starting as we often get samples that have been ruined during sample preparation.
- Do the samples contain high amounts of interfering compounds (e.g. PEG, Tween, or other polymers), or salts that needs removal?
- Who will analyze the data? In many projects this is a major bottleneck, both GC-MS and LC-MS data are highly complicated and require training and experience to handle. For scientific projects, it is expected that you have the responsibility to do data analysis.
- How is the experimental design? Does the set include appropriate controls (blanks), and how much should the method be quantitatively validated?
- For submission, all samples must be labelled with printed labels, printed on our label printers.
Please use the Sample Submission Form.
Contract researchWe run a significant number of contract research projects for industry e.g.: i) identification unknown compounds in samples (e.g. fermentation broths or from bio-fuels projects); ii) analysis of mycotoxins, Bacillus toxins (e.g. cereulide and surfactin) and other secondary metabolites; iii) determination of mycotoxin production potential of industrial strains (1); iv) analysis of mycotoxins in food, feed, and raw products (2, 3); and v) aid for secondary metabolite pathway discovery and characterization (5, 6).
Links
- Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography–UV–mass spectrometry methodology, in ScienceDirect
- Dereplication of Microbial Natural Products by LC-DAD-TOFMS, in ACS Publications
- Aggressive dereplication using UHPLC–DAD–QTOF: screening extracts for up to 3000 fungal secondary metabolites, in Analytical and Bioanalytical Chemistry
- Simultaneous determination of ochratoxin A, mycophenolic acid and fumonisin B2 in meat products, in Analytical and Bioanalytical Chemistry
- Involvement of a Natural Fusion of a Cytochrome P450 and a Hydrolase in Mycophenolic Acid Biosynthesis, in American Society for Microbiology
- Accurate prediction of secondary metabolite gene clusters in filamentous fungi, in PNAS
Lorem ipsum
Employees
View a list of employees associated with the platform
Core Manager
Aaron John Christian Andersen Metabolomics Core Manager Phone: +45 45256138 ajca@dtu.dk